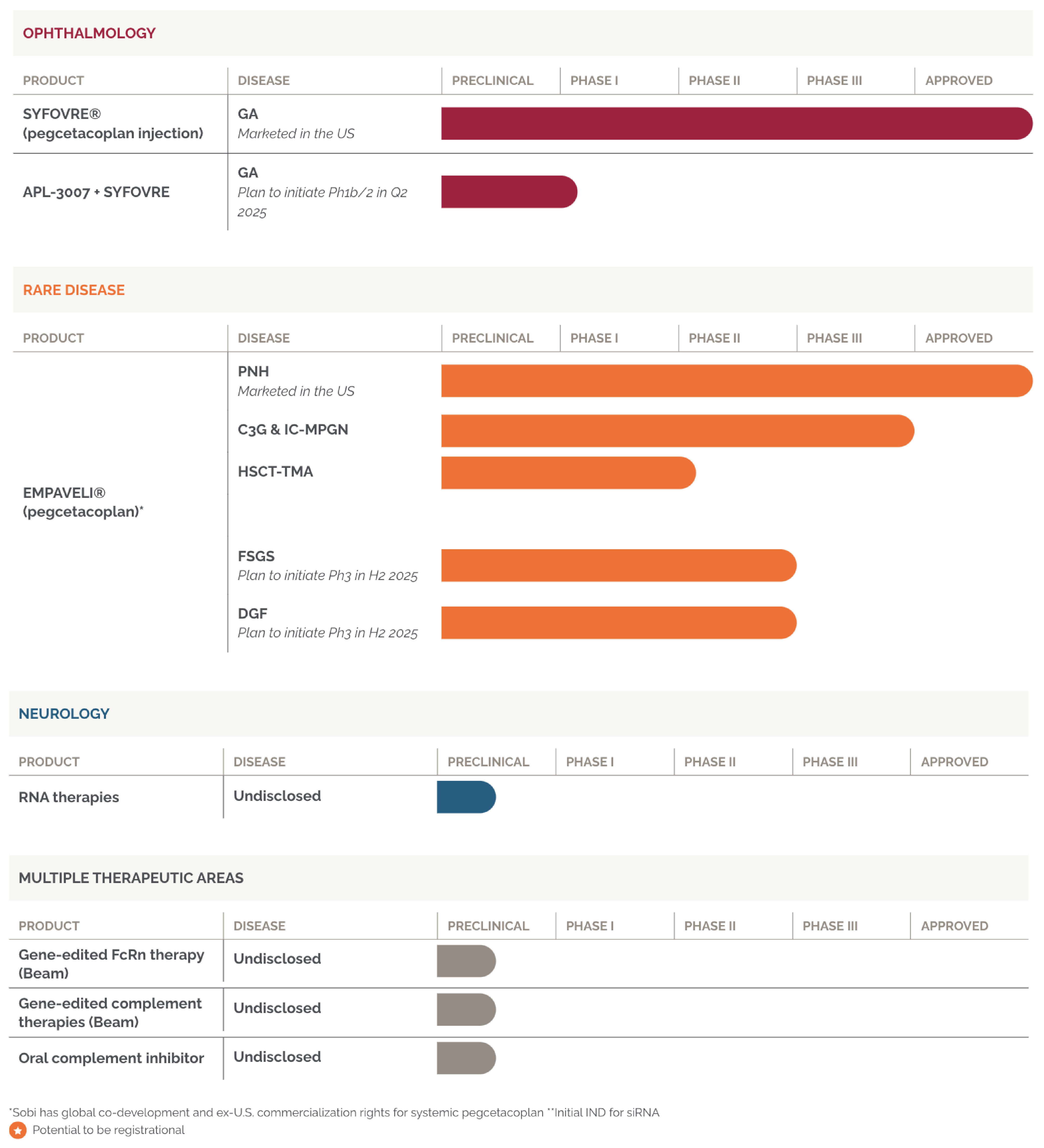
Our Pipeline
Apellis’ efforts are focused on developing complement immunotherapies. We believe that this approach can result in broad inhibition of the principal pathways of the complement system and has the potential to effectively control a broad range of serious diseases.
The safety and efficacy of the agents for the indications under investigation have not been established. There is no guarantee that the outcome of these studies will result in approval by a Health Authority.